Authors:Arash Salavitabar, MD and Darren Berman, MD
How I do it: Tips, Tricks, and Techniques
A PICS Society education series
The Use of CardioMEMS™ Implantable Hemodynamic Monitor in Congenital Heart Disease
The CardioMEMS™ HF System (Abbott, Abbott Park, IL) is a FDA-approved implantable hemodynamic monitor that measures pulmonary artery (PA) pressures. The FDA approval has been expanded to patients with NYHA Class II or III heart failure who have either been hospitalized for heart failure in the previous year and/or have elevated natriuretic peptides. CardioMEMS™ implantation has been described in patients with congenital heart disease and heart failure.1-3 The radiopaque device is 15mm long x 3mm wide x 2mm thick (Figure 1) and consists of a coil and capacitor, which forms an electrical circuit that wirelessly transmits PA pressures that are measured via resonant frequency shifts to a remote, web-based system when the patient places an external antenna (embedded within a pillow) underneath his/her back. The sensor anchors within a branch PA via proximal and distal nitinol loops.
CardioMEMS™ implantation is relatively straightforward, however certain underlying patient anatomies can provide challenges. The most common example in the literature is a patient who has undergone an atrial switch operation (Mustard or Senning), which can require tortuous intracardiac catheter turns and thus the use of a long delivery sheath.
- Access: Femoral or internal jugular vein
- Imaging: Pre-procedural CTA imaging can be used to understand distal pulmonary artery diameter measurements. The target implant vessel should be within the lower lobe of either lung and in a vessel that is directed posteriorly toward the patient's back. This position is to facilitate communication with the Patient Electronics System, or "patient pillow", on which the patient lays to transmit pressures as an outpatient. According to the CardioMEMS™ IFU, the vessel diameter should be >7 mm where body of sensor will be placed and 5 - 8 mm where the distal loop of sensor will be placed.4 We have found that a distal target diameter of 7-10 mm works well.
- Important considerations and reasons for potentially avoiding CardioMEMS™ implantation:
- Sheaths: The device delivery system fits through a 12-French sheath, however it can be delivered through an 11-French Terumo Pinnacle sheath (Terumo Medical Corporation, Somerset, NJ).
- Catheters: Balloon wedge catheter to obtain wire position
- Devices: CardioMEMS™ device and catheter, In-hospital calibration system
- Other: Interventional (stiff) 0.018" wire, 12-French long sheath if desired for angiography and/or to assist in tracking the device in a patient with tortuous course (e.g., atrial switch post-operative anatomy)
-
Details of the individual tip and technique:
Pitfalls to avoid
CardioMEMS™ sensor implantation is feasible and safe in patients with complex congenital heart disease and amenable distal pulmonary vasculature. This can be performed through a short sheath or with the assistance of a long sheath. Delivery of the sensor is very intuitive but may require additional techniques to avoid sensor movement following release in some cases. The sensor can be quickly calibrated to directly measured invasive pulmonary artery pressures and used for outpatient hemodynamics as needed.
- Bradley EA, Berman D, Daniels CJ. First implantable hemodynamic monitoring device placement in single ventricle Fontan anatomy. Catheter Cardiovasc Interv. 2016;88:248-252. https://doi.org/10.1002/ccd.26498.
- Bradley EA, Jassal A, Moore-Clingenpeel M, Abraham WT, Berman D, Daniels CJ. Ambulatory Fontan pressure monitoring: results from the implantable hemodynamic monitor Fontan feasibility cohort (IHM-FFC). Int J Cardiol. 2018;284:22-27. https://doi.org/10.1016/j.ijcard.2018.10.081.
- Salavitabar A, Bradley EA, Chisolm JL, et al. Implantable pulmonary artery pressure monitoring device in patients with palliated congenital heart disease: Technical considerations and procedural outcomes. Catheter Cardiovasc Interv. 2019;1–10. https://doi.org/10.1002/ccd.28528.
- St. Jude Medical I. CARDIOMEMS™ HF System PA sensor and delivery system product highlights. Retrieved from https://www.sjm.com/en/professionals/resources-and-reimbursement/technical-resources/cardiac-rhythm-management/heart-failure-monitoring-system/pa-pressure-monitoring/cardiomems-hf-system-pa-sensor-and-delivery-system?clset=af584191-45c9-4201-8740-5409f4cf
- Rali AS, Shah Z, Sauer A, Gupta K. Late migration of a CardioMEMS™ wireless pulmonary artery hemodynamic monitoring sensor. Circ Hear Fail. 2017;10(4):e003948. https://doi.org/10.1161/circheartfailure.117.003948.
Figures with legends:
Figure 1
CardioMEMS™ device (a and b) tethered to the delivery catheter, and delivery system with release mechanism (c), shown prior to device release. Star, proximal anchoring nitinol loop; asterisk, distal anchoring nitinol loop. Reproduced with permission from John Wiley & Sons, Inc.3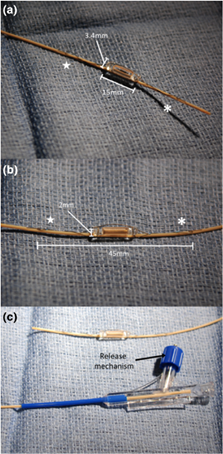
Figure 2
The "calibration wand" (a) is placed underneath the patient using fluoroscopic guidance (b) immediately following device release. The transcatheter pressure (c) is entered into the attached computer system, which calibrates a baseline pressure waveform (d). Reproduced with permission from John Wiley & Sons, Inc.3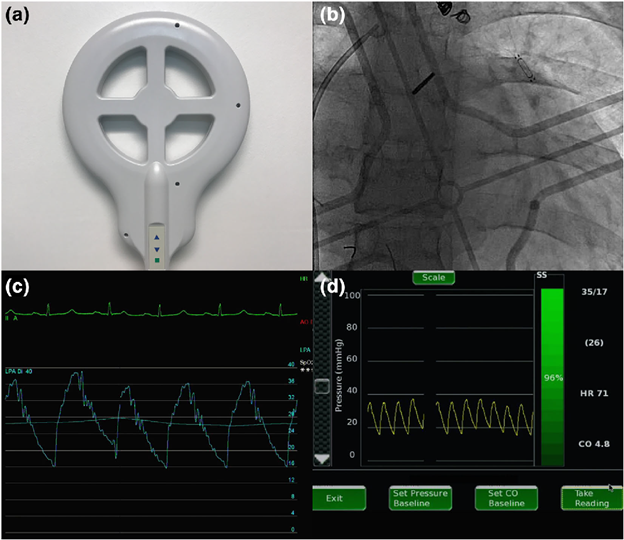
Video 1:
Biplane transcatheter angiography to identify the target vessel in a patient with a Fontan operation.
Video 2:
Release of the sensor with the distal left pulmonary artery.
Video 3:
Post-implantation angiogram confirming final device position.